Which of the Following Best Describes an Ionic Bond
Asked Sep 10 2016 in Environmental Atmospheric Sciences by harsh23. Which statement best describes how an ionic bond forms.
Which of the following best describes ionic bonding.
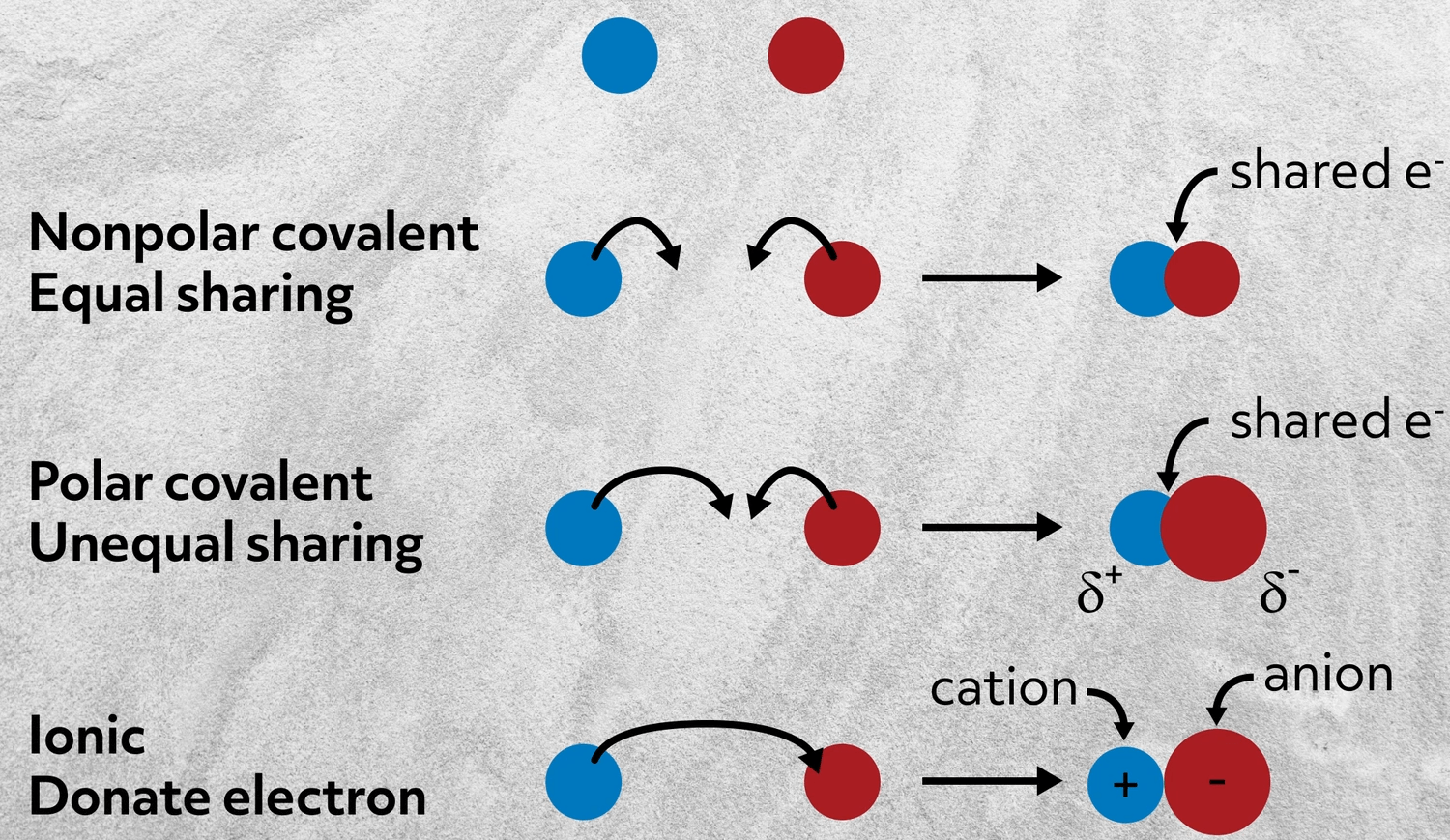
. The attraction between two charged atoms a relatively weak bond in an aqueous solution. In the bond market the bond demanders are the _____ and the bond suppliers are the _____. Each atom contributes an equal number of electrons towards the bond formation.
An ionic bond between oppositely charge ions. The sharing of a pair of electrons between two atoms a relatively weak bond. Which of the following situations best describes an ionic bond.
The bond between the carbons in the molecule H₂CCH₂ is stronger than the bond between the carbons in HCCH. All of the above. Ionic bonds form only between metals and nonmetals.
The transfer of valence electrons between atoms to make them neutral. The number of electrons lossed or gained by each ion. Which one of the following.
Which of the following is not a property of an ionic compound. Which of the following statements best describes an ionic bond. Biology 19092019 0620 lisnel Which best describes how an ionic bond forms.
Thats because metals want to give up electrons and nonmetals want to gain electrons. Which of the following pairs of elements would most likely form a salt. A bond that forms when two atoms with neutral charges come close to one another.
1 on a question. Which of these best describes an ionic bond. An ionic bond is the force of attraction that holds together oppositely charged ions.
A salt forms in the reaction of barium with chlorine. A bond that forms when the electrons of two different atoms repel each other. A bond that forms when the electrons of two different atoms repel each other.
An ionic bond between oppositely charge ions. What is the most likely formula unit of this salt. Which of the following situations best describes an ionic bond.
The repulsion of ions due to the transfer of valence electrons d. Which of the following best describes an ionic bond. A bond that forms when electrons are transferred from one atom to another.
Two nonmetals that share electrons. A bond that forms when electrons are transferred from one atom to another. The bond dissociation energy to break 4 bond s in 1 mole of CH₄ molecules is.
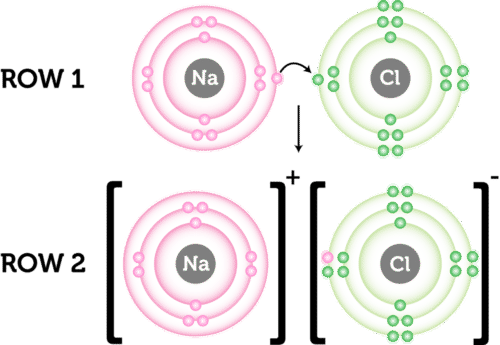
It forms when atoms of a metal transfer electrons. The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds. The attraction of ions due to the transfer of valence electrons b.
A found in most compounds in organisms B involves sharing of electrons C involves gaining or losing neutrons D involves gaining or losing electrons A saturated lipid contains a A more oxygen atoms than hydrogen atoms B no double or triple bonds in the fatty acids chains C. Which of the following best describes an ionic bond. The transfer of electrons from the metal to the non-metal.
A metal that shares electrons with a nonmetal. A bond that forms when two atoms with neutral charges come close to one another. It takes energy to remove valence electrons from an atom and form a positive ion.
View the full answer. Which of the following best describes a ionic bond. The transfer of electrons from the metal to the non-metal.
A bond that forms when one atoms. Which of the following best describes ionic bonding. The sharing of valence electrons between two or more neutral atoms c.
One atom gains an electron while the other atom loses an electron and an. Which of the. A two atoms sharing a set of electrons.
The answer is d An ionic bond involves a metal that transfers one or more electrons to a nonmetal. Which of the following elements when combined with fluorine would produce the most ionic bond. Two atoms each give up an electron and an electrostatic force attracts them.
B two atoms exchanging a set of electrons. Which of the following best describes an ionic bond. C one atom giving up some of its electrons to another atom.
A force that keeps two large molecules together an attraction that occurs between two nonmetals a force that holds two oppositely charged ions together an electromagnetic attraction that occurs between two metals. Which of the following describes an ionic bond quizlet. A metal that transfers one or more electrons to a nonmetal.
Two metals that exchange electrons. The covalent bond is a bond formed when two atoms share one or more electron pairs. The bond dissociation energy to break 1 hydrogen-carbon bond s in 1 mol of HCCH molecules is.
The sharing of a pair of electrons between two atoms a relatively strong bond. Electrostatic force attracts them. Which of the following describes an ionic bond.
An ionic bond is the force of attraction that holds together positive and negative ions.
Solved Which Of The Following Best Describes An Ionic Bond Chegg Com

Comments
Post a Comment